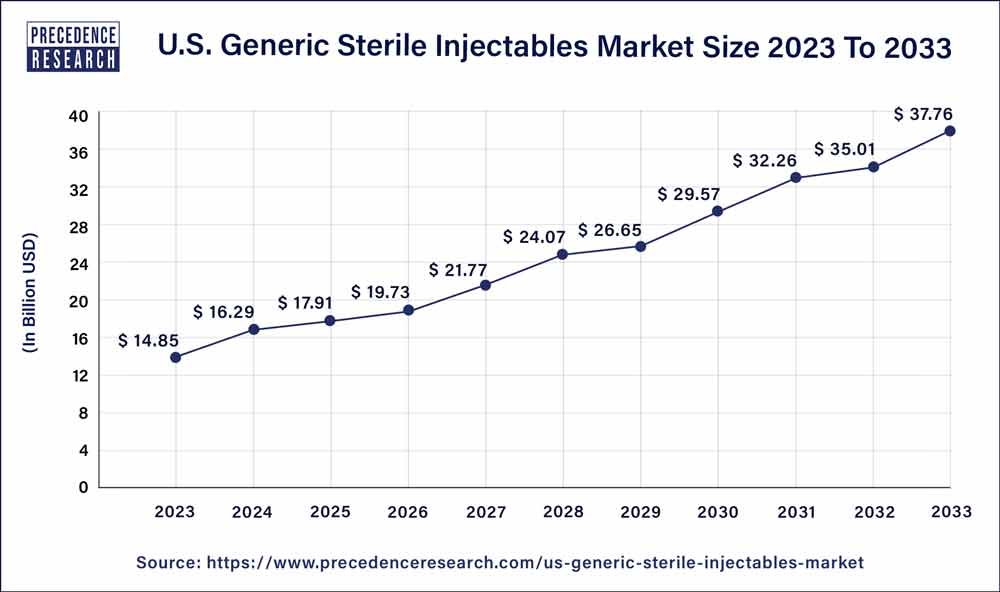
The US generic sterile injectables market size was valued at USD 14.85 billion in 2023 and is projected to surpass around USD 37.76 billion by 2033, growing at a CAGR of 9.79% from 2024 to 2033.
Key Takeaways
- By product type, the monoclonal antibodies segment dominated the market with the largest share in 2023.
- By product, the vaccine segment is expected to grow at a rapid rate over the forecast period.
- By therapeutic application, the cancer segment held the dominating share in 2023 in the US generic sterile injectables market.
- By distribution channel, the hospitals segment held the largest share of the market in 2023. The segment is observed to sustain the position during the forecast period.
The market research report on the US generic sterile injectables market provides a comprehensive analysis of various key aspects. It includes the definition, classification, and application of US generic sterile injectables products. The report examines the development trends, competitive landscape, and industrial chain structure within the industry. Furthermore, it presents an overview of the industry, analyzes national policies and planning, and offers insights into the latest market dynamics and opportunities at a global level.
Get a Sample: https://www.precedenceresearch.com/sample/3778
US Generic Sterile Injectable Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 9.79% |
| U.S. Market Size in 2023 | USD 14.85 Billion |
| U.S. Market Size by 2033 | USD 37.76 Billion |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Product Type, By Therapeutic Application, and By Distribution Channel |
Read More: Redox Flow Battery Market Size, Share, Share, Report By 2033
The report presents the volume and value-based market size for the base year 2022 and forecasts the market’s growth between 2023 and 2032. It estimates market numbers based on product form and application, providing size and forecast for each application segment in both global and regional markets.
Focusing on the global US generic sterile injectables market, the report highlights its status, future forecasts, growth opportunities, key market players, and key market regions such as the United States, Europe, and China. The study aims to present the development of the US generic sterile injectables market by considering factors like Year-on-Year (Y-o-Y) growth, in addition to Compound Annual Growth Rate (CAGR). This approach enables a better understanding of market certainty and the identification of lucrative opportunities.
Regarding production, the report investigates the capacity, production, value, ex-factory price, growth rate, and market share of major manufacturers, regions, and product types. On the consumption side, the report focuses on the regional consumption of US generic sterile injectables products across different countries and applications.
Buyers of the report gain access to verified market figures, including global market size in terms of revenue and volume. The report provides reliable estimations and calculations for global revenue and volume by product type from 2023 to 2032. It also includes accurate figures for production capacity and production by region during the same period.
The research includes product parameters, production processes, cost structures, and data classified by region, technology, and application. Furthermore, it conducts SWOT analysis and investment feasibility studies for new projects.
This in-depth research report offers valuable insights into the US generic sterile injectables market. It employs an objective and fair approach to analyze industry trends, supporting customer competition analysis, development planning, and investment decision-making. The project received support and assistance from technicians and marketing personnel across various links in the industry chain.
The competitive landscape section of the report provides detailed information on xx market competitors. It includes company overviews, financials, revenue generation, market potential, research and development investments, new market initiatives, global presence, production sites, production capacities, strengths and weaknesses, product launches, product range, and application dominance. However, the data points provided only focus on the companies’ activities related to the US generic sterile injectables market.
Prominent players in the market are expected to face tough competition from new entrants. Key players are targeting acquisitions of startup companies to maintain their dominance. The report
Reasons to Purchase this Report:
- Comprehensive market segmentation analysis incorporating qualitative and quantitative research, considering the impact of economic and policy factors.
- In-depth regional and country-level analysis, examining the demand and supply dynamics that influence market growth.
- Market size in USD million and volume in million units provided for each segment and sub-segment.
- Detailed competitive landscape, including market share of major players, recent projects, and strategies implemented over the past five years.
- Comprehensive company profiles encompassing product offerings, key financial information, recent developments, SWOT analysis, and employed strategies by major market players.
US Generic Sterile Injectables Market Companies
- Pfizer
- Mylan
- Sandoz
- 3M
- Par Pharmaceutical, Inc.
- Hikma Pharmaceuticals PLC
- Gland Pharma Limited
- CIVICA
- Ascendia Pharmaceuticals
- Merck & Co., Inc.
Segments Covered in the Report
By Product Type
- Monoclonal Antibodies
- Cytokines
- Insulin
- Peptide Hormones
- Vaccines
- Immunoglobulin
- Blood Factors
- Antibiotics
- Others
By Therapeutic Application
- Cancer
- Diabetes
- Cardiovascular Disease
- Central Nervous System
- Musculoskeletal System
- Others
By Distribution Channel
- Hospitals
- Drug Stores
- Retail Pharmacies
- Others
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on US Generic Sterile Injectables Market
5.1. COVID-19 Landscape: US Generic Sterile Injectables Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. US Generic Sterile Injectables Market, By Product Type
8.1. US Generic Sterile Injectables Market Revenue and Volume, by Product Type, 2024-2033
8.1.1 Monoclonal Antibodies
8.1.1.1. Market Revenue and Volume Forecast (2021-2033)
8.1.2. Cytokines
8.1.2.1. Market Revenue and Volume Forecast (2021-2033)
8.1.3. Insulin
8.1.3.1. Market Revenue and Volume Forecast (2021-2033)
8.1.4. Peptide Hormones
8.1.4.1. Market Revenue and Volume Forecast (2021-2033)
8.1.5. Vaccines
8.1.5.1. Market Revenue and Volume Forecast (2021-2033)
8.1.6. Immunoglobulin
8.1.6.1. Market Revenue and Volume Forecast (2021-2033)
8.1.7. Blood Factors
8.1.7.1. Market Revenue and Volume Forecast (2021-2033)
8.1.8. Antibiotics
8.1.8.1. Market Revenue and Volume Forecast (2021-2033)
8.1.9. Others
8.1.9.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 9. US Generic Sterile Injectables Market, By Therapeutic Application
9.1. US Generic Sterile Injectables Market Revenue and Volume, by Therapeutic Application, 2024-2033
9.1.1. Cancer
9.1.1.1. Market Revenue and Volume Forecast (2021-2033)
9.1.2. Diabetes Therapeutic Application
9.1.2.1. Market Revenue and Volume Forecast (2021-2033)
9.1.3. Cardiovascular Disease
9.1.3.1. Market Revenue and Volume Forecast (2021-2033)
9.1.4. Central Nervous System
9.1.4.1. Market Revenue and Volume Forecast (2021-2033)
9.1.5. Musculoskeletal System
9.1.5.1. Market Revenue and Volume Forecast (2021-2033)
9.1.6. Others
9.1.6.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 10. US Generic Sterile Injectables Market, By Distribution Channel
10.1. US Generic Sterile Injectables Market Revenue and Volume, by Distribution Channel, 2024-2033
10.1.1. Hospitals
10.1.1.1. Market Revenue and Volume Forecast (2021-2033)
10.1.2. Drug Stores
10.1.2.1. Market Revenue and Volume Forecast (2021-2033)
10.1.3. Retail Pharmacies
10.1.3.1. Market Revenue and Volume Forecast (2021-2033)
10.1.4. Others
10.1.4.1. Market Revenue and Volume Forecast (2021-2033)
Chapter 11. US Generic Sterile Injectables Market, and Trend Forecast
11.1. U.S.
11.1.1. Market Revenue and Volume Forecast, by Product Type (2021-2033)
11.1.2. Market Revenue and Volume Forecast, by Therapeutic Application (2021-2033)
11.1.3. Market Revenue and Volume Forecast, by Distribution Channel (2021-2033)
Chapter 12. Company Profiles
12.1. Pfizer
12.1.1. Company Overview
12.1.2. Product Offerings
12.1.3. Financial Performance
12.1.4. Recent Initiatives
12.2. Mylan
12.2.1. Company Overview
12.2.2. Product Offerings
12.2.3. Financial Performance
12.2.4. Recent Initiatives
12.3. Sandoz
12.3.1. Company Overview
12.3.2. Product Offerings
12.3.3. Financial Performance
12.3.4. Recent Initiatives
12.4. 3M
12.4.1. Company Overview
12.4.2. Product Offerings
12.4.3. Financial Performance
12.4.4. Recent Initiatives
12.5. Par Pharmaceutical, Inc.
12.5.1. Company Overview
12.5.2. Product Offerings
12.5.3. Financial Performance
12.5.4. Recent Initiatives
12.6. Hikma Pharmaceuticals PLC
12.6.1. Company Overview
12.6.2. Product Offerings
12.6.3. Financial Performance
12.6.4. Recent Initiatives
12.7. Gland Pharma Limited
12.7.1. Company Overview
12.7.2. Product Offerings
12.7.3. Financial Performance
12.7.4. Recent Initiatives
12.8. CIVICA
12.8.1. Company Overview
12.8.2. Product Offerings
12.8.3. Financial Performance
12.8.4. Recent Initiatives
12.9. Ascendia Pharmaceuticals
12.9.1. Company Overview
12.9.2. Product Offerings
12.9.3. Financial Performance
12.9.4. Recent Initiatives
12.10. Merck & Co., Inc.
12.10.1. Company Overview
12.10.2. Product Offerings
12.10.3. Financial Performance
12.10.4. Recent Initiatives
Chapter 13. Research Methodology
13.1. Primary Research
13.2. Secondary Research
13.3. Assumptions
Chapter 14. Appendix
14.1. About Us
14.2. Glossary of Terms
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/