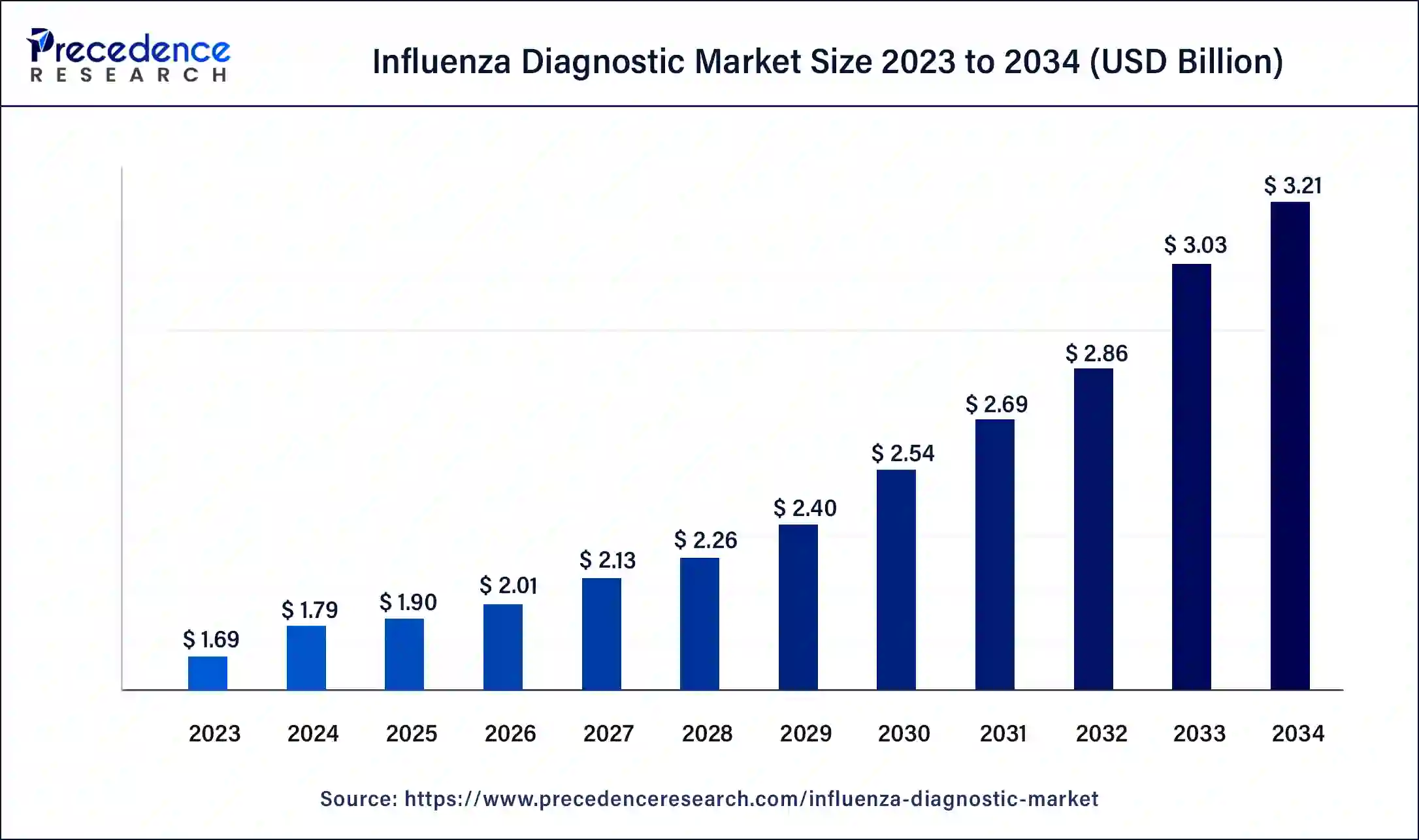
The global influenza diagnostic market size was estimated at USD 1.69 billion in 2023 and is predicted to hit around USD 2.97 billion by 2033, growing at a CAGR of 5.80% from 2024 to 2033.
Key Points
- North America dominated the market with the highest market share of 34% in 2023.
- Asia Pacific is observed to be the fastest-growing region in the market during the forecast period.
- By test type, the RIDT segment has contributed the largest share of 31% in 2023.
- By end-use, the hospitals segment dominated the market with the major market share of 48% in 2023.
The Influenza Diagnostic Market is a pivotal segment of the healthcare industry dedicated to the diagnosis and management of influenza infections. Influenza, commonly known as the flu, is a highly contagious viral respiratory illness caused by influenza viruses. It poses a significant public health challenge worldwide, leading to substantial morbidity, mortality, and economic burden. Accurate and timely diagnosis of influenza is crucial for appropriate patient management, infection control measures, and public health interventions. The Influenza Diagnostic Market encompasses a wide range of diagnostic tests, including rapid antigen tests, molecular assays, serological tests, and viral culture techniques, designed to detect and identify influenza viruses in clinical specimens.
Get a Sample: https://www.precedenceresearch.com/sample/3965
Growth Factors:
Several factors are driving the growth of the Influenza Diagnostic Market. Firstly, the seasonal nature of influenza outbreaks fuels the demand for diagnostic tests during peak flu seasons, leading to increased market uptake. Additionally, the growing awareness about the importance of early diagnosis and prompt treatment of influenza among healthcare providers and the general population contributes to market growth. Moreover, advancements in diagnostic technologies, such as the development of rapid molecular assays and point-of-care tests, have facilitated quick and accurate detection of influenza viruses, driving market expansion. Furthermore, the increasing prevalence of influenza strains with pandemic potential, such as avian influenza and swine flu, underscores the need for robust diagnostic capabilities, further boosting market growth.
Region Insights:
The Influenza Diagnostic Market exhibits regional variations influenced by factors such as healthcare infrastructure, prevalence of influenza, regulatory environment, and technological advancements. North America holds a significant share of the market, driven by high healthcare spending, widespread adoption of advanced diagnostic technologies, and proactive public health measures. Europe follows closely, characterized by strong regulatory frameworks, well-established healthcare systems, and increasing awareness about influenza prevention and control. The Asia Pacific region is witnessing rapid market growth attributed to the rising incidence of influenza, expanding healthcare access, and increasing investments in healthcare infrastructure. Additionally, emerging economies in Latin America, Africa, and the Middle East are experiencing a growing demand for influenza diagnostics due to improving healthcare facilities and rising awareness about infectious diseases.
Influenza Diagnostic Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 5.80% |
| Global Market Size in 2023 | USD 1.69 Billion |
| Global Market Size by 2033 | USD 2.97 Billion |
| U.S. Market Size in 2023 | USD 400 Million |
| U.S. Market Size by 2033 | USD 710 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Test Type and By End-use |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Influenza Diagnostic Market Dynamics
Drivers:
Several drivers propel the growth of the Influenza Diagnostic Market. One primary driver is the increasing incidence of influenza worldwide, driven by factors such as population growth, urbanization, globalization, and environmental changes. Additionally, the growing emphasis on early diagnosis and treatment of influenza to reduce disease severity, complications, and transmission rates stimulates market growth. Furthermore, the implementation of vaccination programs and public health initiatives aimed at influenza prevention and control contributes to the expansion of the diagnostic market. Moreover, technological advancements enabling the development of highly sensitive, specific, and rapid diagnostic tests enhance market prospects by improving diagnostic accuracy and efficiency.
Opportunities:
The Influenza Diagnostic Market presents numerous opportunities for stakeholders across the healthcare continuum. Firstly, there is immense scope for innovation and product development in diagnostic technologies to enhance test sensitivity, specificity, and turnaround time. Secondly, expanding market penetration in emerging economies offers significant growth opportunities, fueled by rising healthcare expenditure, increasing disease awareness, and improving healthcare infrastructure. Moreover, partnerships, collaborations, and strategic alliances between diagnostic companies, healthcare providers, and public health agencies can facilitate market expansion and address unmet diagnostic needs. Furthermore, the integration of digital health solutions, such as telemedicine and remote monitoring, into influenza diagnostics presents novel avenues for enhancing patient access and healthcare delivery.
Challenges:
Despite substantial growth prospects, the Influenza Diagnostic Market faces several challenges that warrant attention. One major challenge is the variability of influenza viruses and the emergence of new strains, necessitating continuous surveillance and adaptation of diagnostic tests to detect evolving viral subtypes accurately. Additionally, the presence of asymptomatic carriers and mild cases of influenza poses challenges for diagnosis and surveillance efforts, potentially leading to underestimation of disease burden and inadequate public health response. Moreover, regulatory hurdles and reimbursement issues in certain regions may hinder market growth by delaying product approvals and limiting reimbursement coverage for diagnostic tests. Furthermore, the COVID-19 pandemic has impacted the Influenza Diagnostic Market by diverting healthcare resources, disrupting supply chains, and altering healthcare-seeking behavior, underscoring the need for resilient diagnostic strategies in the face of emerging infectious threats.
Read Also: Mammography Systems Market Size to Attain USD 6.22 Bn by 2033
Recent Developments
- In May 2023, Hologic Inc. declared that the Panther Fusion assay had received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This assay is a molecular diagnostic test that can identify and distinguish between four common respiratory viruses that can present with similar clinical symptoms: influenza A (flu A), influenza B (flu B), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and respiratory syncytial virus (RSV).
- In January 2023, the healthcare goods and solutions firm 2San released a dual kit for SARS-CoV-2 and Influenza A+B. The OTC kit was introduced to ease the strain on healthcare facilities due to the continued demand on the National Health Service (NHS) following the epidemic.
Influenza Diagnostic Market Companies
- 3M Company
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- Meridian Bioscience, Inc.
- Quidel Corporation
- SEKISUI Diagnostics
- Thermo Fisher Scientific, Inc.
- Hologic, Inc.
- F. Hoffmann-La Roche Ltd
- SA Scientific Ltd
Segments Covered in the Report
By Test Type
- RIDT
- RT-PCR
- Cell Culture
- Others
By End-use
- Hospitals
- POCT
- Laboratories
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/