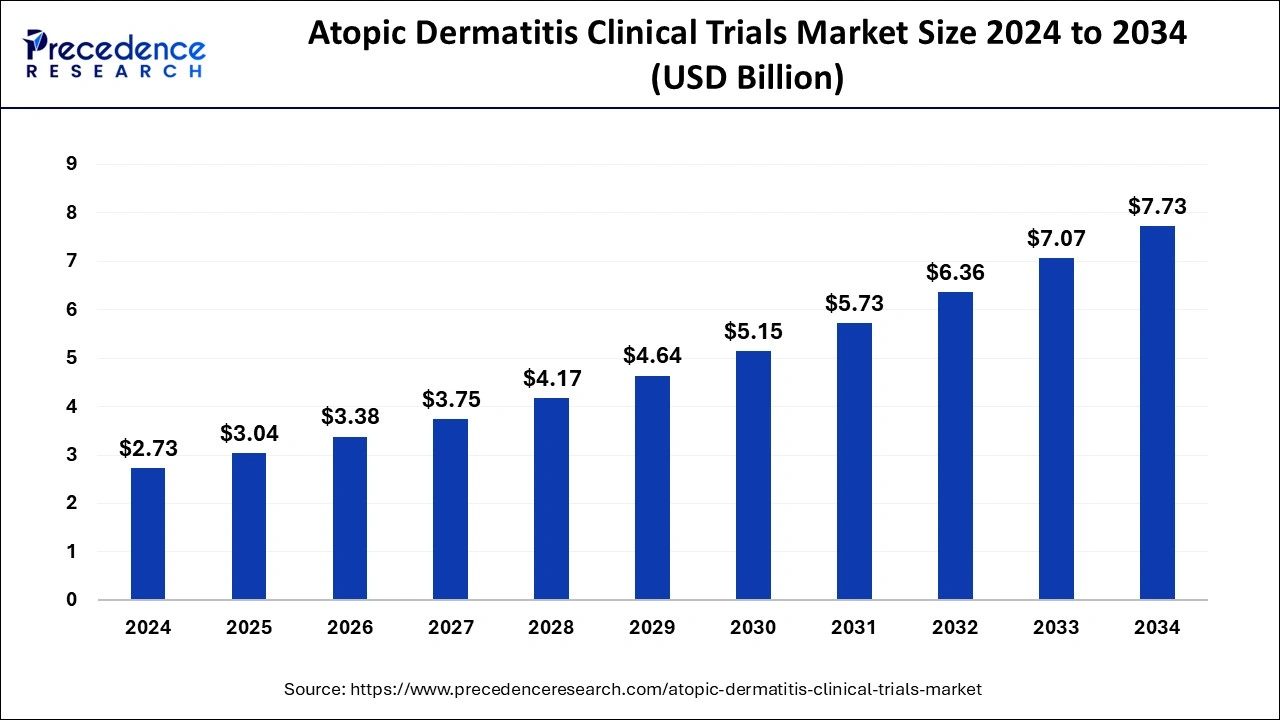
The global atopic dermatitis clinical trials market size was estimated at USD 2.46 billion in 2023 and is anticipated to reach around USD 7.07 billion by 2033, expanding at a CAGR of 11.14% from 2024 to 2033.
Key Points
- North America has contributed more than 37% of market share in 2023.
- Asia-Pacific is estimated to expand the fastest CAGR between 2024 and 2033.
- By molecule type, the large molecules segment has held the largest market share of 54% in 2023.
- By molecule type, the small molecules segment is anticipated to grow at a remarkable CAGR of 12.5% between 2024 and 2033.
- By study design, the interventional trials segment has generated over 72% of market share in 2023.
- By study design, the observational trials segment is expected to expand at the fastest CAGR over the projected period.
- By phase, the phase II segment has accounted more than over 48% of market share in 2023.
- By phase, the phase III segment is expected to expand at the fastest CAGR over the projected period.
Atopic Dermatitis (AD) is a chronic inflammatory skin condition characterized by itching, redness, and scaling. It commonly affects children but can persist or onset in adulthood. With the rising prevalence of AD globally, there has been a surge in clinical trials aimed at developing effective treatments. The Atopic Dermatitis Clinical Trials Market encompasses a broad spectrum of research endeavors, including drug trials, therapy evaluations, and diagnostic innovations. These trials play a crucial role in advancing our understanding of AD pathogenesis and improving patient outcomes.
Get a Sample: https://www.precedenceresearch.com/sample/4021
Growth Factors:
Several factors contribute to the growth of the Atopic Dermatitis Clinical Trials Market. Firstly, the increasing prevalence of AD worldwide has heightened the demand for novel therapies and interventions. Additionally, advancements in medical research techniques and technologies have facilitated the development of more targeted and efficacious treatments. Moreover, the growing awareness among healthcare professionals and patients regarding the impact of AD on quality of life has spurred investments in clinical research.
Region Insights:
Clinical trials for atopic dermatitis are conducted across various regions globally, reflecting the widespread prevalence of the condition. North America, Europe, and Asia Pacific are among the key regions witnessing significant activity in AD clinical research. In North America, particularly in the United States, a robust regulatory framework and substantial investments in healthcare infrastructure drive the conduct of numerous clinical trials. Europe, with its well-established research institutions and collaborations, also contributes significantly to the AD clinical trials market. Meanwhile, Asia Pacific is emerging as a promising region for clinical research, fueled by growing healthcare expenditure and rising awareness of AD.
Atopic Dermatitis Clinical Trials Market Scope
| Report Coverage | Details |
| Growth Rate from 2024 to 2033 | CAGR of 11.14% |
| Global Market Size in 2023 | USD 2.46 Billion |
| Global Market Size by 2033 | USD 7.07 Billion |
| U.S. Market Size in 2023 | USD 640 Million |
| U.S. Market Size by 2033 | USD 1,830 Million |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Molecule Type, By Study Design, and By Phase |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Atopic Dermatitis Clinical Trials Market Market Dynamics
Drivers:
The Atopic Dermatitis Clinical Trials Market is driven by several factors. One of the primary drivers is the unmet medical need for effective and safe treatments for AD. The current therapeutic options often provide only partial relief or come with significant side effects, highlighting the urgency for innovative therapies. Additionally, the growing understanding of AD’s complex pathophysiology has led to the identification of novel drug targets and therapeutic approaches, driving research efforts. Furthermore, regulatory incentives and collaborations between pharmaceutical companies, academic institutions, and government agencies play a crucial role in accelerating clinical trial activities.
Opportunities:
The Atopic Dermatitis Clinical Trials Market presents numerous opportunities for stakeholders across the healthcare spectrum. For pharmaceutical companies, investing in AD clinical trials offers the potential for developing blockbuster drugs and capturing a significant share of the dermatology market. Academic institutions and research organizations can leverage these trials to advance scientific knowledge and train the next generation of researchers. Healthcare providers stand to benefit from access to cutting-edge treatments and diagnostic tools emerging from clinical research, ultimately improving patient care and outcomes.
Challenges:
Despite the opportunities, the Atopic Dermatitis Clinical Trials Market also faces several challenges. One of the significant challenges is patient recruitment and retention, particularly in long-term trials. AD clinical trials often require large cohorts and extended follow-up periods, making recruitment and retention efforts resource-intensive. Additionally, ensuring diversity and representation across demographics poses a challenge, as certain populations may be underrepresented in clinical research. Moreover, regulatory complexities and evolving guidelines can create hurdles for trial sponsors, necessitating meticulous planning and compliance measures.
Read Also: Air Traffic Control (ATC) Equipment Market Size, Trend, Report 2033
Recent Developments
- In October 2023, LEO Pharma disclosed the favorable outcome of the DELTA 3 trial. This Phase 3 extension trial assessed delgocitinib cream, an investigational topical pan-Janus kinase (JAK) inhibitor, for potential treatment in adults with moderate to severe chronic hand eczema (CHE). This positive result bolstered the company’s pipeline value and is expected to facilitate the commercialization of the product.
- In May 2023, Dermavant Sciences, Inc. unveiled encouraging results from ADORING 1, Phase 3 studies investigating the efficacy and safety of topical VTAMA (tapinarof) cream 1% in adults and pediatric subjects as young as 2 years old with moderate to severe atopic dermatitis. These findings provided the company with a route to commercial success, expansion in the market, competitive differentiation, and increased investor support.
Atopic Dermatitis Clinical Trials Market Companies
- Pfizer Inc.
- Sanofi SA
- Regeneron Pharmaceuticals, Inc.
- AbbVie Inc.
- Novartis International AG
- Eli Lilly and Company
- GlaxoSmithKline plc
- Johnson & Johnson
- Leo Pharma A/S
- Dermira, Inc. (acquired by Eli Lilly and Company)
- Galderma S.A.
- AnaptysBio, Inc.
- Dermavant Sciences, Inc.
- LEO Pharma A/S
- Amgen Inc.
Segments Covered in the Report
By Molecule Type
- Small Molecules
- Large Molecules
By Study Design
- Interventional
- Observational
By Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/